Science: Chemistry:
Morphology Controlled MoO3/SiO2 Catalyst for Hydrodesulfurization
Seung Hyun Cho and Dr. Jin-Wook Ha+
Department of Chemical Engineering, Soonchunhyang University
ABSTRACT
Several
series of morphologically controlled MoO3/SiO2 catalysts
were prepared, characterized, and tested for hydrodesulfurization (HDS) of dibenzothiophene
(DBT) activity. Molybdenum surface loaded with 4.0 atoms Mo/nm2 was
prepared as sintered hexagonal and sintered orthorhombic, as well as a novel
"well dispersed hexagonal" phase. Characterization by XRD, Raman, and
O2 chemisorption results reveals that the dispersion of MoO3 over
silica depends on the final MoO3 phase in the order of: sintered
hexagonal < sintered orthorhombic < dispersed hexagonal phase. Temperature
programmed reduction (TPR) results show that both bulk and dispersed
microcrystalline of MoO3 reduce to MoO2 at 650C and to Mo metal at 1000C. HDS of DBT was performed in a
differential reactor at 30 atm over the temperature range 350-500C. Activity of MoO3/SiO2
toward HDS of DBT is proportional to dispersion.
Key
Words: Morphology of MoO3/SiO2,
Hydrodesulfurization of Dibenzothiophene
1.
INTRODUCTION
Continual
effort is put into characterization of the types of Mo oxide species which
exist over various supports, their stability, and the optimal preparation
techniques to obtain them. A recent
series of comprehensive works performed with Raman and UV over a number of
supports and supported oxides and with a wide array of preparation procedures
have synthesized many of these concepts into a general theory [1-7]. First, it
was postulated that the surface species of molybdenum present in the air
exposed, hydrated catalyst is only a function of pH in the hydrated layer.
Second, the amount of material deposited in well dispersed form depends on the
number of reactive hydroxyl groups present. In all of the referenced work, MoO3
produced by a calcination at temperatures in excess of 450°C, which produces the well known and
characterized orthorhombic MoO3 phase. The present work with silica
supported MoO3 has centered on investigating, and exploiting the
behavior of the little studied hexagonal phase of MoO3. The
morphology of silica supported catalysts was fully characterized by using XRD,
Raman, BET, and TPR. Activity was tested by hydrodesulfurization
(HDS) of dibenzothiophene (DBT) at 30 atm over the temperature range from 350
to 500C.
The
current study seeks to further characterize the highly dispersed hexagonal
phase and then to establish structure-function relationships for all types of
highly loaded catalysts. It is desired to develop a comprehensive understanding
of the highly loaded catalysts which will complement the current high degree of
understanding of low loading catalyst.
2.
EXPERIMENTAL
2.1 Catalyst Preparation
The
support material used for the preparation of this study was Aerosil 380
(surface area is 380m2/gm), manufactured by Degussa. To densify the
silica, it was wetted with deionized water, dried overnight at room temperature
(24°C),
and then dried at 110°C for 12h in a muffle furnace. Ammonium
hepta-molybdate (IV) tetrahydrate (AHM), (NH4)6Mo7O24.4H2O,
supplied by Aldrich Chemical Company Inc. was used as a precursor. Surface
loading of MoO3 over silica
was 4 atoms Mo/nm2. Loadings were prepared by physically mixing the
desired amount of dry support and precursor, and then adding deionized water to
reach incipient wetness. Acid or base impregnations were conducted with
concentrated nitric acid (2.5N) or ammonium hydroxide (5N) in place of
deionized water. After impregnation the samples were thoroughly stirred and
dried in air overnight.
Table
1. Supported precursor and MoO3
patterns resulting from various preparation conditions for supported MoO3/SiO2
|
Precursor |
Impregnation (Dry T) |
Phase of MoO3 formed |
Calcination Temp.(time) |
Phase of MoO3 formed |
|
AHM/SiO2 |
Acida (25C) |
Hexagonal (NH3)0.15.MoO3.0.5 H2O/SiO2 |
300C (2h) in air |
Hexagonal (sintered wrt precursor) |
|
AHM/SiO2 |
Waterb (25C) |
AHM (NH4)6Mo7O24.4H2O/ SiO2 |
500C (2h) in air |
Orthorhombic (sintered wrt precursor) |
|
AHM/SiO2 |
Basec (25C) |
Triclinic (NH4)2Mo2O7/SiO2 |
300C (2h) in air |
Dispersed Hexagonal |
a Nitric acid,HNO3(2.5N),b Deionized water,H2O(pH=5),c Ammonium hydroxide,NH4OH(5N).
The
final distribution of material appeared homogeneous with the use of watchglasses.
Calcinations were performed in air in a standard muffle furnace. A summary of
sample treatments and final phases of supported MoO3 is given in Table 1. Three different MoO3 phase over
silica will be referred as "SO" for sintered orthorhombic, "SH"
for sintered hexagonal and "DH" for dispersed hexagonal phase of MoO3/SiO2.
2.2 Catalyst Characterization
A
Siemens D 5000 X-ray powder diffractometer was used to identify the various
crystalline phases. Measurements were done at 50 kV, 30 mA, and suitable two
theta range (8-33°), theta step (0.05°) and count
time (3 sec). Raman spectroscopy of catalysts (wet and calcined samples) under
ambient conditions were obtained with an Ar+ laser (Spectra Physics
Model 171) by utilizing about 10-40 mW of the 514.5 nm line for excitation.
Temperature programmed reduction (TPR) was carried at the temperature range
between 400 and 1000°C with 5°C increment.
Reducing gas was 4 vol % of H2 in N2, flowing at 16.8
sccm. Dispersion of MoO3 over SiO2 was estimated by low temperature
(25°C) O2 chemisorption. Activity
for hydrodesulfurization of dibenzothiophene (DBT) was performed in a
differential reactor at 30 atm over the temperature range between 350 and 500C.
3. RESULTS
AND DISCUSSION
The
effect of the precursor pH (acidic, neutral and basic precursor) and
calcination temperature was studied by XRD and results shown in Figure 1.
Acidic precursor and 300°C calcinations,
sintered hexagonal phase of MoO3 was observed. Sintered
Orthorhombic MoO3 was observed with
neutral precursor and 500°C calcinations.
And for basic precursor and 300°C calcination
samples, both hexagonal and orthorhombic phase of MoO3 was observed.
The
crystallite size of the fraction of crystallites observable by XRD was estimated
from the Scherrer equation to be; 137 Å (SH), 88 Å (SO), and 59 Å (DH), using the
full width at half maximum (FWHM) of the unsupported H(210) (hexagonal phase of
MoO3 crystal) and
R(110) (orthorhombic phase of MoO3 crystal)
phases as standards for instrumental broadening. However, since the integrated
intensities of the DH are well below those of the sintered phases a good
portion of the material also exists as very small(less than 40 Å particles
beyond the detection limit of XRD). These could be amorphous or
microcrystalline. No material was lost through volatilization in this series; a
high degree of crystallinity can be reestablished in the dispersed samples by
subsequent high temperature treatment.
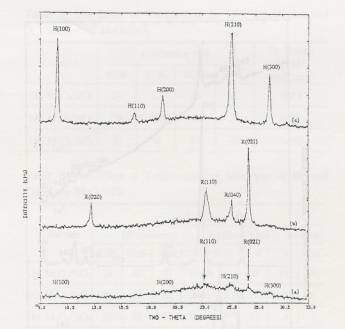
Figure 1. XRD
patterns of calcined 4 atoms Mo/nm2 supported MoO3/SiO2:
(a) basic precursor, 300C calcined in air (DH), (b) neutral
precursor, 500C calcined in air (SO), (c) acid
precursor, 300C calcined in air (SH).
The
Raman spectra of supported MoO3/SiO2 catalysts
under ambient conditions are shown in figure 2. Trends are almost same to XRD
results, a much lesser degree of crystallinity for the well-dispersed hexagonal
sample compared to the sintered hexagonal and orthorhombic.
The
crystalline MoO3 peak is much
more sensitive in Raman than the polymolybdate (Mo7O246-)
peak; even though the intensity is high, the relative amount is low. Wachs'
group has reported that the calcined samples exhibit the very weak Raman
features due to the surface molybdenum oxide species, Mo7O246-
and/or Mo8O264-. It means that dispersed
hexagonal sample actually has less MoO3 crystalline
and more surface molybdenum oxide species, Mo7O246-.
O2
uptake of sulfided MoO3/SiO2 catalysts is given in Table
2. Results also show that dispersion degree of DH is much larger than those of
sintered catalysts (SH and SO).
Table
2. O2 uptake
of 4 atoms Mo/nm2 supported sulfided MoO3/SiO2
catalysts
|
Catalysts |
O2 / MoS2 (mmol / g) |
O2 / Mo (mmol / mmol ) |
|
SH MoO3/SiO2 SO MoO3/SiO2 DH MoO3/SiO2 |
93.86 163.61 289.60 |
0.0150 0.0262 0.0464 |
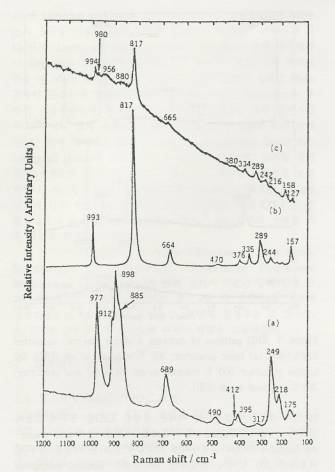
Figure 2. Raman
spectra of calcined 4 atoms Mo/nm2 supported MoO3/SiO2:
(a) acid precursor, 300C calcined in air (SH), (b) neutral
precursor, 500C calcined in air (SO), (c) basic
precursor, 300C calcined in air (DH).
An
initial series of experiments with physically mixed samples were performed to
clarify the interpretation of TPR results. TPR profiles of silica supported MoO3/SiO2 and physical
mixture of MoO3+SiO2
were
shown in figure 3. TPR results show that MoO3 crystal can be
reduced at two regions and the area of the first peak is always close to 1/2
that of the second one.
XRD
data taken for the impregnated and physically mixed samples from TPR
experiments stopped at 650°C and 1000°C were shown in figures 4 and 5,
respectively. XRD results showed that MoO3 crystal
reduced to MoO2 by low
temperature reuction and displayed the pattern of metallic Mo after TPR to 1000°C.
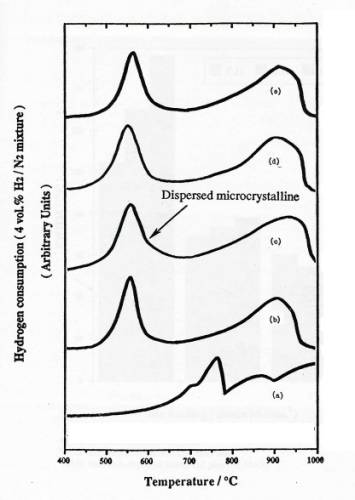
Figure 3. TPR
profiles of samples: (a) bulk MoO3, (b) physically mixed 4 atoms
Mo/nm2 MoO3+SiO2, calcined 4 atoms Mo/nm2
supported MoO3/SiO2: (c) basic precursor, 300C calcined in air (DH), (d) acid
precursor, 300C calcined in air (SH), (e) neutral
precursor, 500C calcined in air (SO).
These
results implies that MoO3 crystal reduce
by two step over silica as follows:
MoO3 (both dispersed
and bulk) + H2 ® MoO2 (TPR up to 650°C)
MoO2 (both dispersed
and bulk) + 2H2 ®
Mo (TPR up to 1000°C)
Mo(VI) + 3H2 ® Mo(0) (overall reaction)
The
percentage conversion of DBT and the selectivity to biphenyl (BP) were given in
Tables 3 and 4. The selectivity to BP was
about 80% at temperature ranges from 350 to 400C. However, selectivity to BP was almost
100% at 500C.
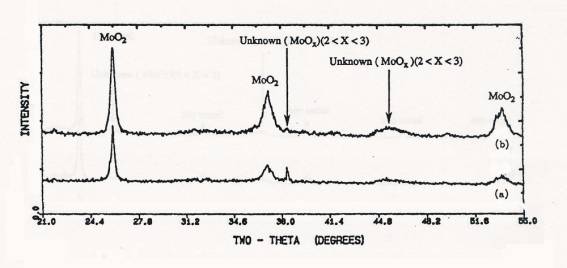
Figure 4. XRD
patterns of samples after TPR upto 650C: (a) calcined 4 atoms Mo/nm2
supported MoO3/SiO2(SO), (b) physically mixed 4 atoms Mo/nm2 MoO3+SiO2.
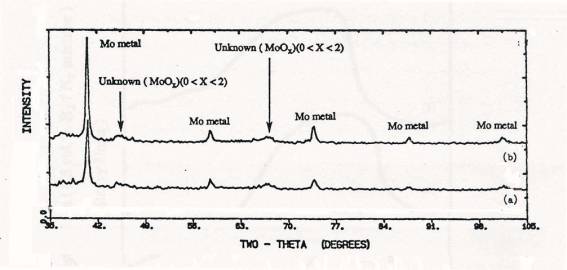
Figure 5. XRD
patterns of samples after TPR upto 1000C: (a) calcined 4 atoms Mo/nm2
supported MoO3/SiO2(SO), (b) physically mixed 4 atoms
Mo/nm2 MoO3+SiO2.
Table 1. Supported precursor and MoO3
patterns resulting from various preparation conditions for supported MoO3/SiO2
Activity
of catalysts toward hydrodesulfurization of DBT is proportional to dispersion
in the order of: sintered hexagonal < sintered orthorhombic < dispersed
hexagonal phase MoO3/ SiO2. TOFs of the
crystalline phases appear higher than that of the dispersed phase. However, the
predominant factor for activity per gram of MoO3 for hydrodesulfurization of DBT is dispersion;
the higher activity of the crystallites does not compensate their lesser
dispersion.
Table 3. The effect of temperature on DBT
conversion and O2 uptake over MoO3/SiO2
|
Catalyst |
Conversion (%) |
O2 uptake O2/Mo(mol/mol) |
|||
|
|
623K |
673K |
723K |
773K |
|
|
SH MoO3/SiO2 |
24 |
27 |
36 |
47 |
0.018 |
|
SO MoO3/SiO2 |
28 |
30 |
42 |
55 |
0.027 |
|
DH MoO3/SiO2 |
32 |
48 |
54 |
74 |
0.048 |
Table 4. The effect of temperature on selectivity
of BP and CHB over MoO3/SiO2
|
Catalyst |
Selectivity (%) |
|||||||
|
|
BP(biphenyl) |
CHB
(cyclohexyl benzene) |
||||||
|
|
623K |
673K |
723K |
773K |
623K |
673K |
723K |
773K |
|
SH MoO3/SiO2 |
79 |
80 |
85 |
91 |
21 |
20 |
15 |
9 |
|
SO MoO3/SiO2 |
81 |
83 |
92 |
98 |
19 |
17 |
8 |
2 |
|
DH MoO3/SiO2 |
82 |
85 |
96 |
99 |
18 |
15 |
4 |
1 |
REFERENCES
1. K. Segawa and W. S. Millman, J. Catal., 77, 221 (1982).
2. L. Wang and W. K. Hall, J. Catal., 77, 232
(1982).
3. F.
J. Gil-Llambias, A. M. Escudey-Castro, and A. Lopez Agudo, J. Catal. 90, 323
(1984).
4. J.
Leyer, M. I. Zaki, Z. Shuxian, and H. Knozinger, Mater. Chem. Phys. 13,
301 (1985).
5. S. J. Moon and S. K. Ihm, Appl. Catal., 42, 307 (1988).
6. A. Datta, J.-W. Ha, and J. R. Regalbuto, J. Catal., 133, 55 (1992).
7. J.-W. Ha, J. of Ind. & Eng. Chemistry, 2(2), 137 (1996).
[ BWW Society Home Page ]
© 2002 The BWW Society/The Institute for the Advancement of Positive Global Solutions